veriseq nipt v2
NovaSeq 6000 Sequencing System is by far our most powerful instrument designed to adapt to your needs. Tenders Electronic Daily TED the European public procurement journal.
Veriseq Nipt Solution V2 Support
Served as technical writing project lead for the release and launch of VeriSeq NIPT Solution v2 an end-to-end solution for non-invasive prenatal testing.
. VeriSeq NIPT Solution v2 Package Insert Translated into Brazilian Portuguese. Prospecto de VeriSeq NIPT Solution v2 - Support Illumina. PDF 1 MB Aug 13 2021.
Instructions for processing samples with the VeriSeq NIPT Solution v2. Why did they start to think about onboarding NIPT technology2. The new version expands the range of chromosomal and sub-chromosomal conditions associated with birth defects that laboratories can screen for.
PDF 1 MB Aug 16 2021. Created edited and updated the Package Insert and Software Guide for the product. Welcome to Immense Discovery Power.
Illumina has launched the VeriSeq NIPT Solution v2 a CE-IVD next-generation sequencing-based approach to noninvasive prenatal testing. The assay provides information about fetal chromosomal status as early as 10. VeriSeq NIPT Solution v2.
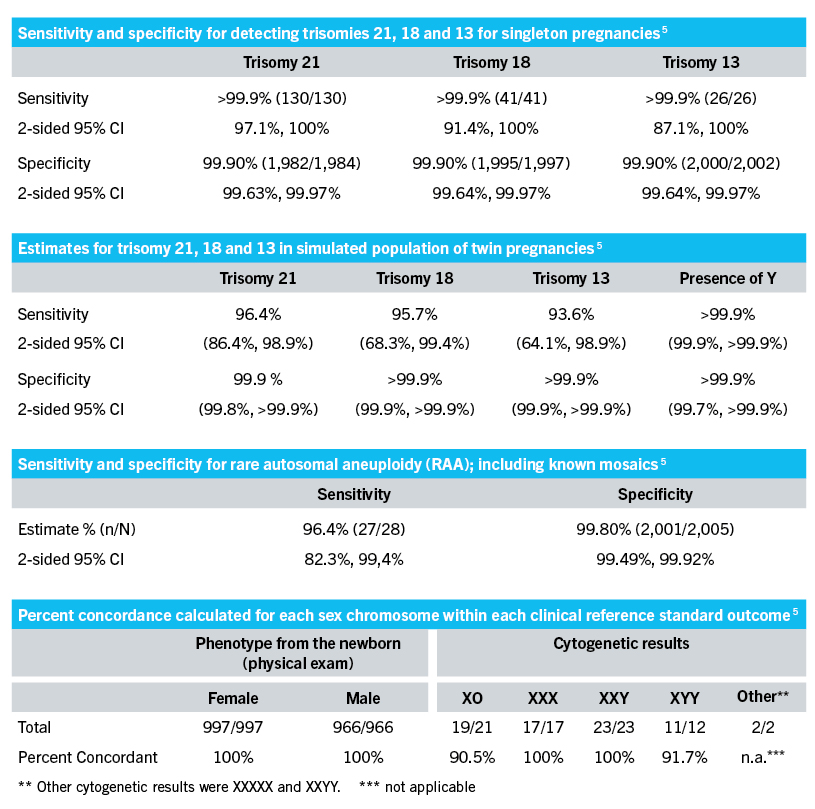
The VeriSeq NIPT Solution v2 is an in vitro diagnostic test intended for use as a screening test for the detection of genome-wide fetal genetic anomalies from maternal peripheral whole blood specimens in pregnant women of at least 10 weeks gestation. Selection Planning Tools. VeriSeq NIPT Solution v2 uses whole-genome sequencing to detect partial duplications and.
VeriSeq NIPT Solution v2 Package Insert 200006957 v00 for Canada. FASTQ files streamed into BaseSpace can be analyzed using the BWA Enrichment App or the Issac Enrichment App v20 and v21 custom manifest workflow. Sequencing with the VeriSeq NIPT Solution v2 enables comprehensive insights reducing the need for invasive tests.
When running the NextSeq in Standalone mode enter the following parameters on the Run Setup Screen. The CE-IVD VeriSeq NIPT Solution v2 is now registered for use in Thailand Vietnam Singapore South Korea Australia New Zealand Israel South Africa and across most countries in Europe. The VeriSeq NIPT Microlab STAR allows a single user to prepare and analyze 48 or 96 samples simultaneously with results in approximately one day compared to two days or longer using other methods.
Hands-free throughput and sample integrity are enabled through powerful features such as eight. Following the simple automated workflow one technician can analyze 24-96 samples in 8 hours with minimal hands-on time. NovaSeq 6000 Sequencing System is by far our most powerful instrument designed to adapt to your needs.
P1 reagents are now available for NextSeq 1000NextSeq 2000 Systems offering added flexibility to meet your projects needs. VeriSeq NIPT Solution v2 Package Insert 1000000078751 v06 1 MB. VeriSeq NIPT Solution v2 provides accurate information about fetal chromosomal status as early as 10 weeks of gestation using a single maternal blood draw.
Tema de la página. Library Prep. VeriSeq NIPT Solution v2 Package Insert Translated into Brazilian Portuguese.
Comprehensive IVD in-lab aneuploidy screening solution providing reagents instruments and software for accurate NIPT results in 26 hours. This noninvasive test provides an option to screen for aneuploidy in all autosomes chromosomes X Y and partial deletions and duplications greater than 7 Mb across the genome. Medical equipments pharmaceuticals and personal care products.
The VeriSeq NIPT Solution v2 is an in vitro diagnostic test intended for use as a screening test for the detection of genome-wide fetal genetic anomalies from maternal peripheral whole blood specimens in pregnant women of at least 10 weeks gestation. Product includes components of library preparation sequencing and analysis. Part Description Dimensions Weight Storage 15071543 VeriSeqNIPTWorkflowTubesandLabels 17cm10cm1cm 67in39in04in 20gr 004 lbs Roomtemperature.
VeriSeq NIPT Solution v2 Package Insert 200006957 v00 for Canada. Set up the run as a dual index paired-end 151-cycle sequencing run. VeriSeq NIPT Solution v2 uses whole-genome sequencing to detect partial duplications and.
Welcome to Immense Discovery Power. Expanded noninvasive prenatal testing looking beyond trisomies T21 T18 and T13. The VeriSeq NIPT Solution v2 is an in vitro diagnostic test intended for use as a screening test for the detection of genome-wide fetal genetic anomalies from maternal peripheral whole blood specimens in pregnant women of at least 10 weeks gestation.
Watch the video to find out why laboratories In Europe have implemented VeriSeq NIPT1. VeriSeq NIPT Solution v2 Package Insert 1000000078751 v06 PDF 1 MB Aug 16 2021. VeriSeq NIPT Solution v2 provides accurate information about fetal chromosomal status as early as 10 weeks of gestation using a single maternal blood draw.
Enhanced Time Savings and Sample Integrity. The VeriSeq NIPT Solution v2 offers a fast three-step workflow for NIPT that generates accurate results in just over one day Table 4. This noninvasive test provides an option.
RevisionHistory Document Date DescriptionofChange Document 1000000067940v06 August 2021 UpdatedEUAuthorizedRepresentativeaddress. NextSeq 10002000 Reagents.
Performance Qualification Praenatest
In Lab Screening With Nipt Turnkey Sample To Results In Your Lab
Illumina Next Generation Genomic Introduce Veriseq Nipt Solution
Veriseq Nipt Solution V2 Comprehensive And Reliable Nipt Solution
Analysis Of Cfdna Using A Modified Illumina Veriseq Non Invasive Download Scientific Diagram
Jcm Free Full Text Strategy For Use Of Genome Wide Non Invasive Prenatal Testing For Rare Autosomal Aneuploidies And Unbalanced Structural Chromosomal Anomalies Html
The Veriseq Nipt Solution Youtube
Veriseq Nipt Solution V2 Comprehensive And Reliable Nipt Solution
In Lab Screening With Nipt Turnkey Sample To Results In Your Lab
Comparison Of Aneuploidy Incidence Between Study Cohorts Download Table
Illumina Introduces Expanded Version Of Veriseq Nipt Solution Offering More Comprehensive Detection Of Rare Chromosomal Conditions Business Wire
Illumina S Noninvasive Prenatal Screening Kit Receives Regulatory Approval In S Korea Business Wire
Illumina Twitter પર Fdesouza Version 2 Of Veriseq Nipt Will Ship In 1h 2019 Adding Karyotype Resolution Across The Genome And Increasing The Number Of Genetic Diseases That Can Be Detected Jpm19
Veriseq Nipt Solution V2 Comprehensive And Reliable Nipt Solution
Veriseq Nipt Solution V2 Comprehensive And Reliable Nipt Solution
Genes Free Full Text Nipt Technique Based On The Use Of Long Chimeric Dna Reads Html

Comments
Post a Comment